In the previous article, we talked about how J.J. Thompson disproved Dalton’s Atomic Theory. In this article, we will discuss the discovery of the nucleus by Rutherford.

The Gold Foil experiment was conducted by emitting alpha particle beams towards a gold foil. Most of the alpha particles from the beam passed through the gold foil, but a few beams were bent or reflected towards the detecting screen. Why did some reflect? And why did others pass? Rutherford concluded that it was because an atom is mainly empty. Not completely empty – mainly empty. Along with defining the nucleus, he also stated its properties.
- Most mass of an atom resides in a nucleus.
- A nucleus in positively charged.
- A nucleus is a very small and extremely dense region.
At this point, you may be wondering why some of the beam bent and others reflected. This is because the some of the beams hit the nucleus on the side, while others hit it straight.
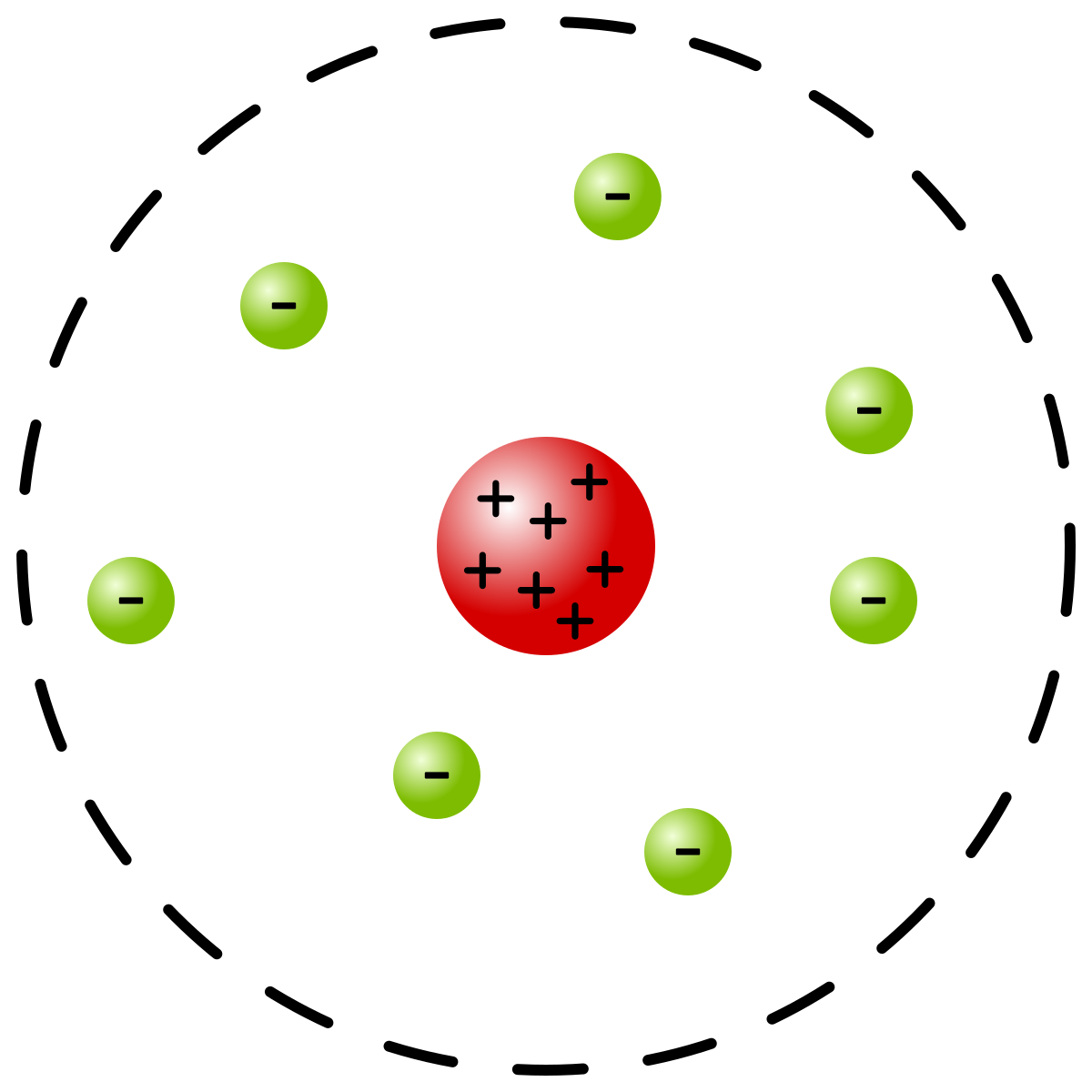
To summarize, Ernest Rutherford discovered the presence and properties of the nucleus through his Gold Foil experiment. In the next article, we will discuss the Bohr model.

